The Auroras: A Light Show
We've all seen the pictures of auroras, and we probably have all asked ourselves, "How is that possible? What is that?!" Maybe these questions were followed by a moment of sheer panic, or the desire to eat some cheese. In any case, this section will answer those burning questions.
The more common name for the Aurora Borealis is the Northern Lights. The Aurora Australis is the term for the auroras that occur in the Southern Hemisphere. At its simplest, an aurora is the result of charged particles that the sun releases into space. When these charged particles plummet into the earth's magnetic field, they are deflected to the poles where they then crash into gas molecules, causing those fantastic colors.
Just like the earth, the sun has an atmosphere and a magnetic field. The hydrogen atoms on the sun are exposed to such high temperatures that their protons and electrons essentially boil away and flow out to space at very high speeds. These, combined with the sun's magnetic field, create what is known as solar wind. Like a galactic bully, solar wind forces earth's magnetic field to change its shape. The magnetic field facing the sun is compressed, while the opposite end is stretched into a long tail called a magnetotail.
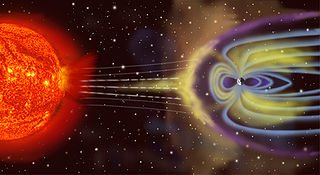
An artist's rendering of Earth's magnetic field. The side of the field facing the sun has been bullied into a compressed shape. The magnetotail is the portion that looks like streamers, or alien appendages. The magnetotail is several times longer than the radius of the earth. (Source)
Being a galactic bully requires an input of energy, and this malevolent energy is stored in the magnetosphere. Eventually, the energy builds up enough, and the stars align just so (metaphorically, not literally) and a voltage passes between Earth's magnetic poles. This voltage shoots electrons toward the poles, where they ascend in the atmosphere until reach the ionosphere.
Unsurprisingly, the ionosphere is filled with (drumroll please)…ions. Ions have a charge. When the charged particles from the sun (mostly electrons) collide with these ions, the ions release light and electrons. Remember, light is given off when atoms fall from one energy level to the next. The color of the light depends on the element involved. Oxygen causes the green-yellow and the deep red colors in auroras. Nitrogen ions produce blue auroras, while nitrogen molecules produce purple-red auroras.
The auroras occur around both poles, in bands centered at each magnetic pole. The band can be between 10 and 1,000 km wide and stray as far as 3,000 km from the magnetic pole. Prime spots for viewing these awesome light shows include Fairbanks, Alaska, Northern Canada, and northern Russia. During intense geomagnetic storms, the auroras can even be spotted as far from the poles as Mexico.

The Aurora Borealis in the North Pole. (Source)